
Inside the ‘front line’ of China’s Covid-19 vaccine development
In some parts of the building, interior furnishing is still underway.
The 20,000 square meter manufacturing plant was built in recent months to produce the Covid-19 vaccine developed by Chinese pharmaceutical company Sinovac Biotech.
CoronaVac, Sinovac’s potential vaccine, is part of China’s efforts to lead the anti-Covid-19 vaccination rollout and to demonstrate the country’s scientific prowess.
Engineers work at Sinovac’s facility in Beijing on April 29.
Scientists around the globe are racing to find ways to stop the virus that has infected 24.3 million people and killed nearly 830,000.
Phase three trials of CoronaVac are underway in Brazil and Indonesia with about 11,000 volunteers.
The biotechnology company began developing the vaccine in late January, less than a week after Wuhan, the initial epicenter of the epidemic in China, was locked down.
CoronaVac uses an old-fashioned approach – using inactivated virus to prompt the body to develop immunity.
Meanwhile, the US is pushing a newer type of vaccine made with genetic material from the virus.
To speed up the pre-clinical process, as researchers develop potential vaccines and test them on animals, Yang said the company conducted several parallel studies to be able to select the best results.
Sinovac also worked closely with China’s pharmaceutical regulators to speed up the approval process.
In May, Sinovac published in the journal Science the results of animal testing, showing that the vaccine effectively created antibodies in mice and rats, as well as primates such as golden monkeys.
But phase three requires large-scale testing with thousands of participants.
Yang said the insignificant number of new infections in China did not meet the necessary conditions for Phase Three testing, so Sinovac reached an agreement with Brazil, the world’s second largest epidemic area with more than 3,
In collaboration with the Butantan Institute in Sao Paulo, the Phase Three trial was launched in late July with the participation of 9,000 volunteers, including all medical staff caring for Covid-19 patients who were not infected with the virus.
Trials are still underway, but Dimas Covas, president of the Butantan Institute, said preliminary data through August 26 showed that CoronaVac stimulated immunity in 97% of people vaccinated with two doses
Earlier this month, Covas said data on the vaccine’s effectiveness could be released in October, potentially launching the approval process in several months.
This month, Sinovac also launched a Phase Three trial in Indonesia with 1,620 volunteers.
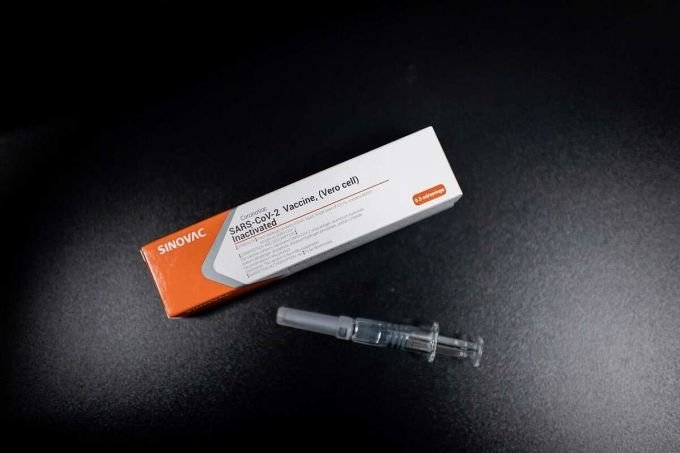
Sinovac’s potential vaccine CoronaVac.
As the global vaccine development race heats up, Yang said that competition among biotech companies is not a major issue because the demand for vaccines is too great for one company to meet.
`Our speed must be faster than the speed of the virus infection instead of competing with others. We want everyone to succeed, so there will be enough supply to protect everyone,` Yang said.
Two other Chinese potential vaccines that have begun phase three trials were developed by state-owned company Sinopharm.
13 companies in China are researching Covid-19 vaccines.
Ivan Hung, an infectious disease expert at the University of Hong Kong’s medical school, said vaccination is key for countries like China, where strict measures have helped keep the rate of nCoV infections down in the population.
Chinese President Xi Jinping has repeatedly urged Chinese scientists to accelerate vaccine research and development.
Vaccines not only help save lives, provide scientific credibility and open up opportunities for economic recovery, they also have significant geopolitical significance.
Successful vaccine development will give China the opportunity to strengthen its global position by positioning itself as a leader in fighting the pandemic, dispelling the claim that it was the place where Covid-19 originated.
Last month, Philippine President Rodrigo Duterte announced that he had asked Xi to give the country priority access to Chinese vaccines.
In an online summit with African leaders in June, Xi pledged that `once the development and deployment of Covid-19 vaccines is completed in China, African countries will be
Earlier this week, Chinese Premier Li Keqiang also said that Beijing would prioritize Southeast Asian countries including Cambodia, Myanmar, Laos, Thailand and Vietnam to access the vaccine.
In China, authorities have authorized `emergency use` of a potential vaccine before the end of late-stage testing.
The vaccine developed by Sinopharm is still in phase three trials in the United Arab Emirates (UAE), Peru, Morocco and Argentina.
Zheng Zhongwei, director of the NHC’s Science and Technology Development Center, said that `emergency use` of unapproved vaccines `in the event of a major public health threat` is appropriate.
However, on August 24, Anthony Fauci, the US’s leading infectious disease expert, warned against authorizing emergency use, explaining that such a step could harm development efforts.
`One of the potential risks if you deploy a vaccine too early is that it will make it difficult, if not block, other vaccines from getting people to participate in trials,` Fauci added.


